Particle size and shape analysis.
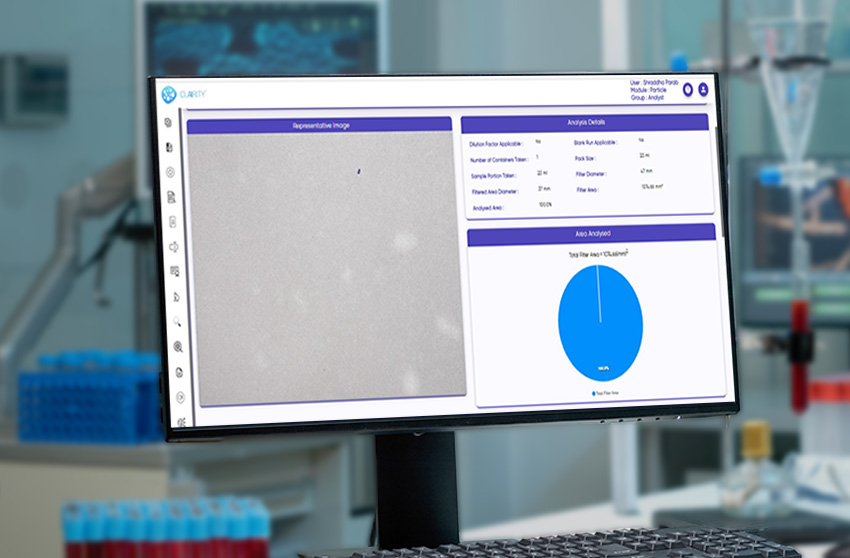
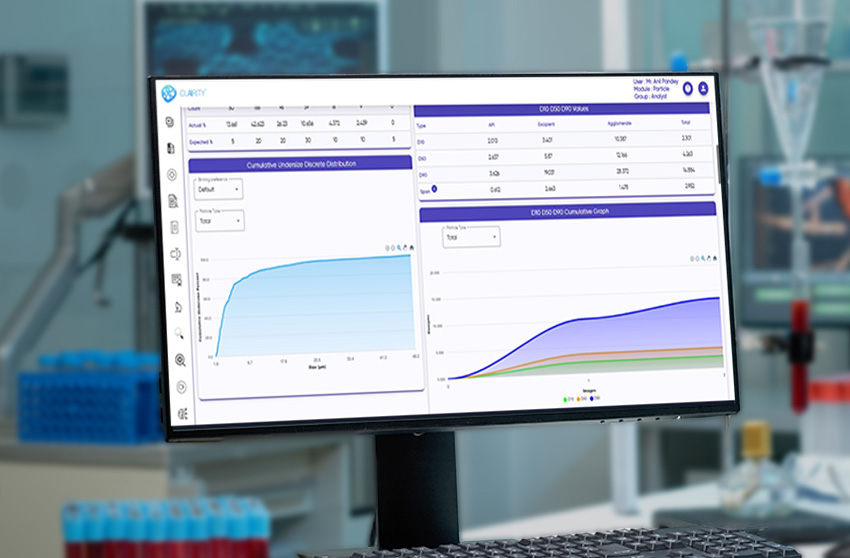
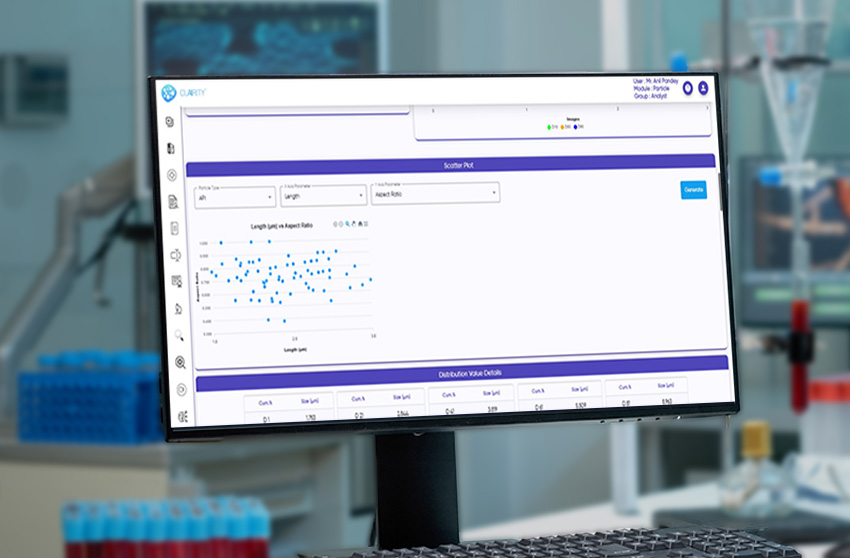
CLAIRITY™ PARTICLE is a high-performance software solution for particle size analysis, shape profiling, and distribution quantification. Purpose-built for pharmaceutical, biotech, and other regulated industries, the platform supports detailed, reproducible microscopic particulate analysis in line with USP and standards.
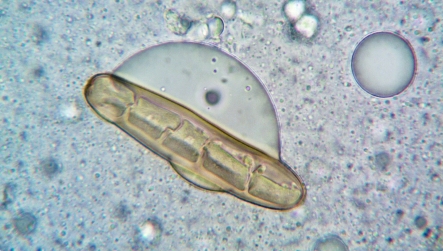
It seamlessly integrates with microscope image analysis software, optical microscopes, SEM, and other imaging systems, enabling precise detection of faint, transparent, or multimodal particles across dosage forms.
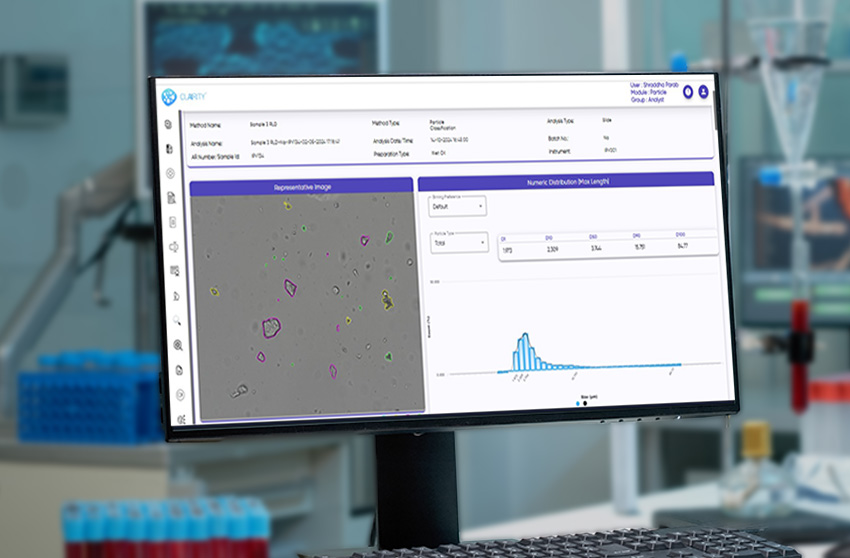
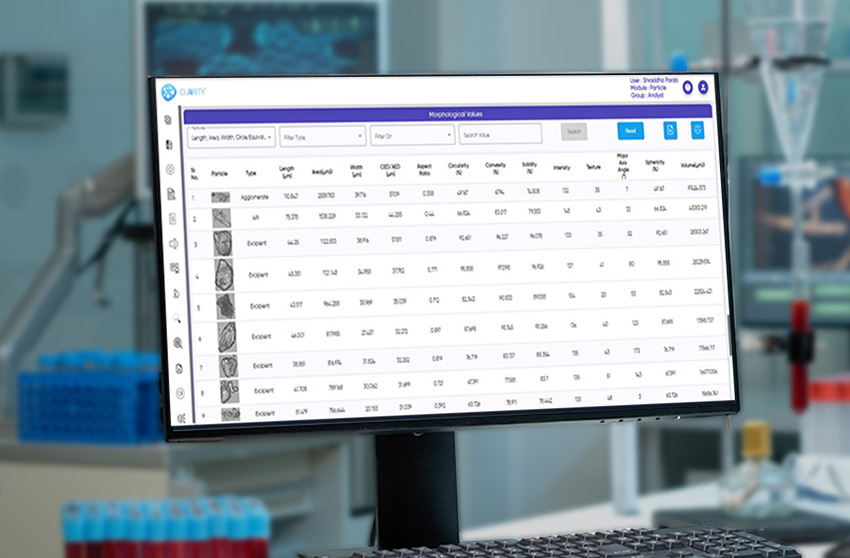
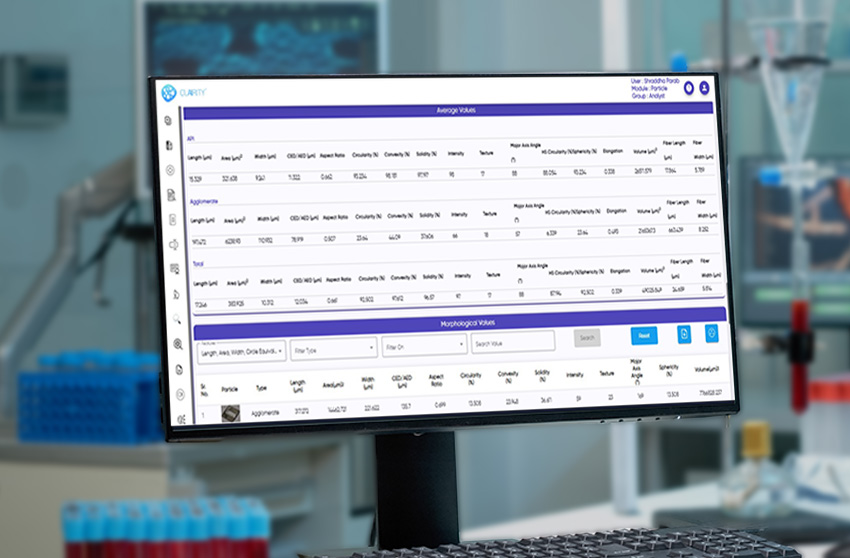
The system fully complies with 21 CFR Part 11, incorporating audit trails, e-signatures, and secure, role-based access for GMP-regulated labs. Its AI engine classifies isolated particles, agglomerates, APIs, excipients, and globules with consistency and speed, while delivering a wide range of morphological parameters such as size, shape, circularity, solidity, texture, and intensity.