Automated microscopy tools.
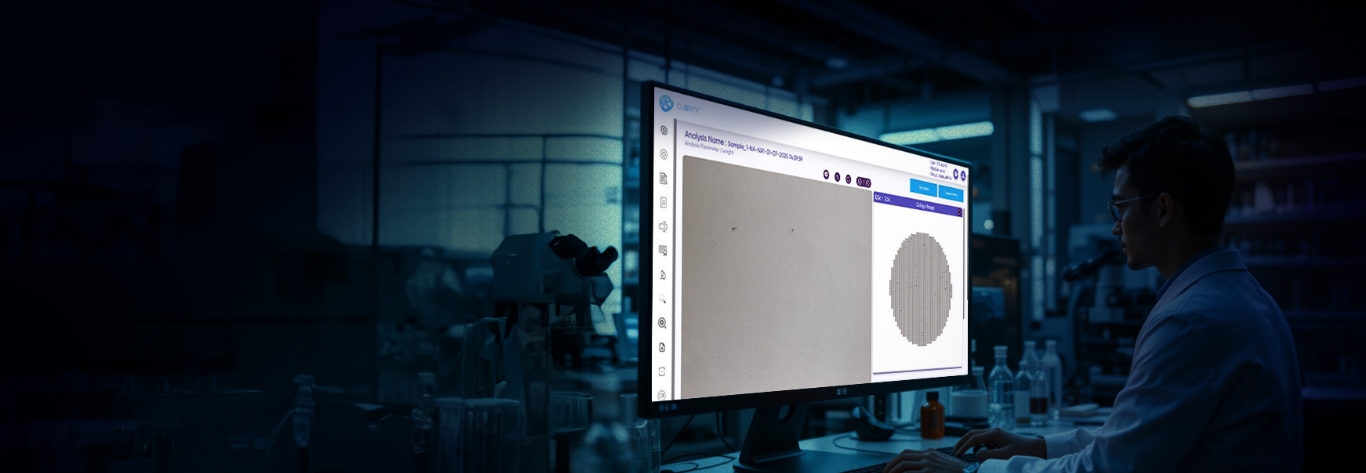
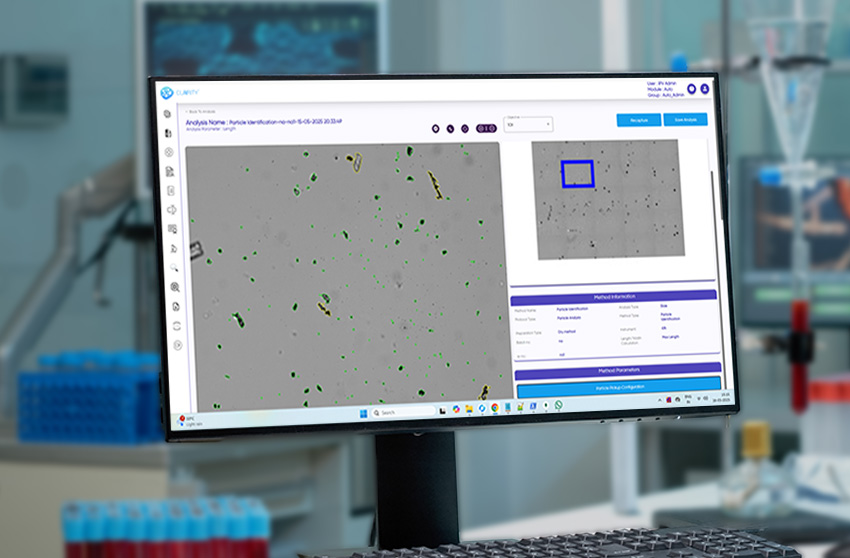
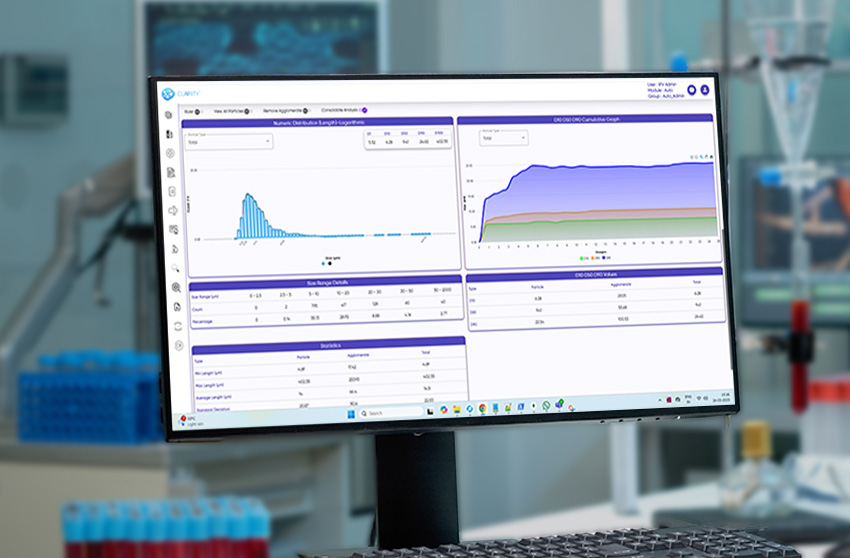
CLAIRITY™ AUTO is an advanced, automation-first solution for intelligent particulate matter detection, designed for pharmaceutical, biotech, and industrial labs where speed, accuracy, and traceability are crucial. The software replaces manual workflows with AI-guided, high-throughput imaging, offering far more reliability than traditional methods like light obscuration.
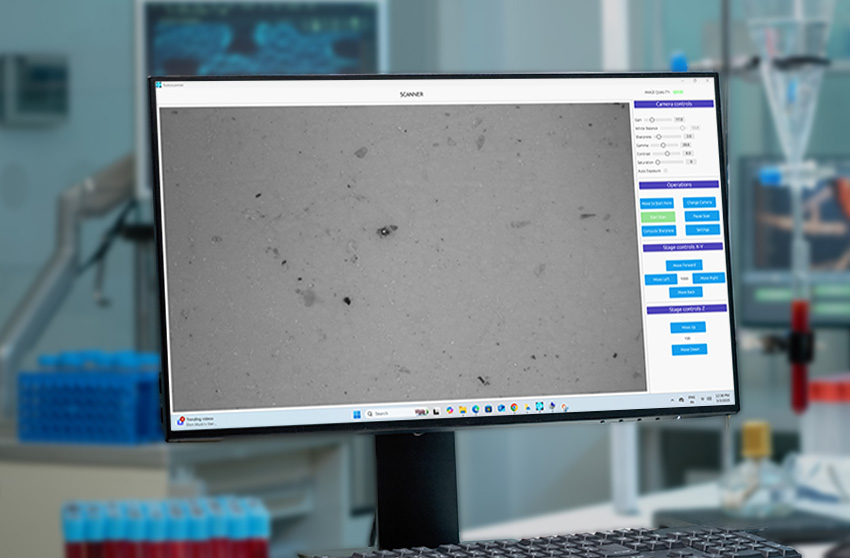
With seamless integration across xyz motorized microscope stages, it supports autofocus, scanning, and stitching, transforming manual microscopy into a consistent, auditable system for microscopic particulate analysis.
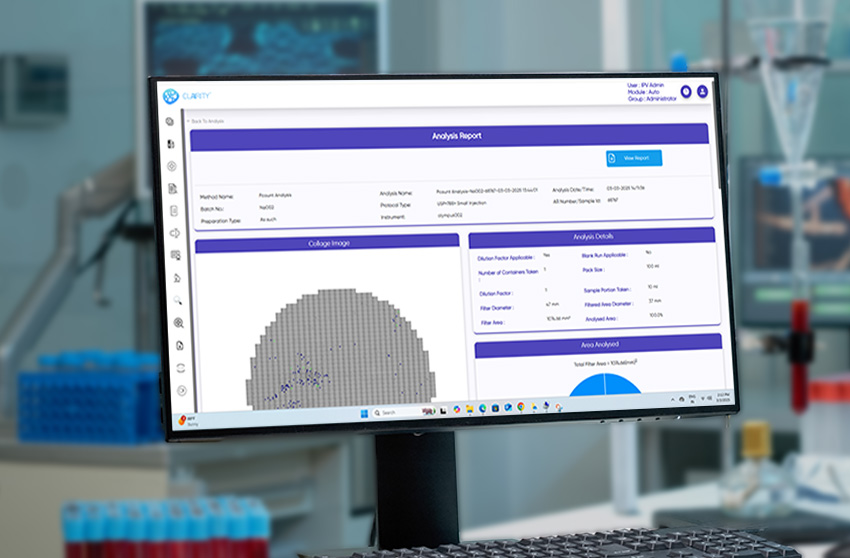
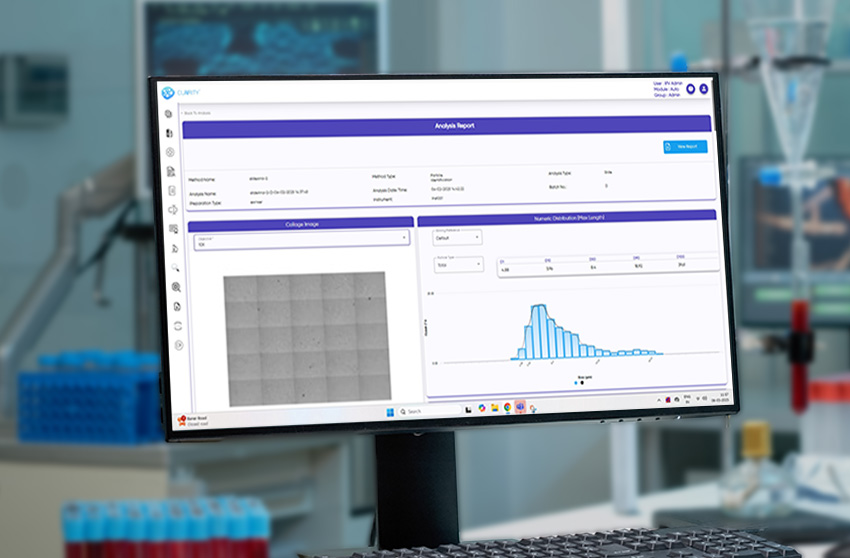
From injectables and ophthalmic solutions to aerosols, MDI, lyophilized products, and foreign matter inspection, CLAIRITY AUTO supports fully automated imaging and analysis pipelines. The platform identifies and classifies a broad spectrum of contaminants—fibers, silicone oil droplets, glass fragments, and particulate matter—without manual intervention, improving reproducibility and reducing analytical fatigue.